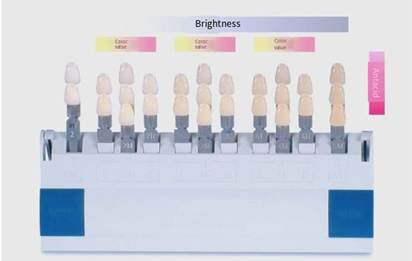
Zirconia All-Ceramic Dentures – The Zirconia Color Section
Home/News/Zirconia All-Ceramic Dentures – The Zirconia Color Section Zirconia All-Ceramic Dentures – The Zirconia Color Section Zirconia all-ceramic restorations are widely

Home/News/Zirconia All-Ceramic Dentures – The Zirconia Color Section Zirconia All-Ceramic Dentures – The Zirconia Color Section Zirconia all-ceramic restorations are widely

Home/News/The Color of Zirconia All-Ceramic Dentures – Color Matching The Color of Zirconia All-Ceramic Dentures – Color Matching Zirconia all-ceramic

Home/New/CE-MDR Certification De Corematrix Co., Ltd Earns EU CE-MDR Certification De Corematrix Co.,Ltd has secured EU Medical Device Regulation (MDR 2017/745)
Home/New/Preventing Fractures in Zirconia All-Ceramic Prostheses: Pre-Sintering Insights and Solutions Preventing Fractures in Zirconia All-Ceramic Prostheses: Pre-Sintering Insights and Solutions Zirconia

Home/New/Preventing Fractures in Zirconia All-Ceramic Prostheses: Pre-Sintering Insights and Solutions Preventing Fractures in Zirconia All-Ceramic Prostheses: Pre-Sintering Insights and Solutions Zirconia

Technical Quality Requirements and Testing Methods of Zirconia Blocks of Decorematrix. (advanced) In the previous article, we introduced part of the
